11
11





How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 10
10




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”
 10
10




Nancy Reading wrote:I've been considering the possibilities of making steel from raw materials. Mostly because I think it would be amusing. Our rock here is basalt, the remnents of an ancient volcano that straddled the Irish sea. I believe I can collect black sand from our local beach as a starting material. Ihave no idea what sort of quality metal I may end up with, but there is a local knife maker who may be interested in making something if I come up with something useable.
"Ah, but a man's reach should exceed his grasp,
Or what's a heaven for?"
Andrea del Sarto by Robert Browning
 8
8




Moderator, Treatment Free Beekeepers group on Facebook.
https://www.facebook.com/groups/treatmentfreebeekeepers/





 9
9




Michael Cox wrote:The techniques that gold prospectors use for gravity separation can help you out. For small batches you could use hand panning, for larger batches you may want to try some kind of sluice.
"Ah, but a man's reach should exceed his grasp,
Or what's a heaven for?"
Andrea del Sarto by Robert Browning
 7
7




Michael Cox wrote:I am interested in the forging aspect of J-tubes, myself. I had recently collected an old hand cranked forge blower in usable condition, and was intending to build a forge pot out of an old heavy truck brake drum from the salvage yard, at least for starts. But, a J-tube rocket (considering I have a collection of salvaged brick, and can get fire brick from a local brick yard) might be a straighter path to the goal, which is to heat ferrous alloys sufficiently that they can be hot worked rather than cold worked. A bit of heat treating, too./quote]
Building a simple forge from bricks is not that difficult. https://permies.com/t/179207/pep-metalworking/Basic-backyard-forge
I used to have a tire rim forge. I traded a small anvil for it some years ago. It had a real coal forge duck nest (fire pot) installed in it and an electric blower with variable speed. The outside of the fire pot was packed with some sort of white clay for insulation. You could build a similar forge using the hand crank blower you have. You might want to create a firepot out of some clay or castable refractory.

I pulled the fire pot out and bult a solid fuel forge around it.
.JPG)
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”
 6
6




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 7
7




How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 9
9




Nancy Reading wrote:Thank you all for your comments!
I'm happy for you to post your button making method here Joshua. Or you can make a new one. It's handy for me to have all the information in one place, but a new thread (link it here please!) is also fine.
.
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”
 7
7




Joshua States wrote:Building a simple forge from bricks is not that difficult. https://permies.com/t/179207/pep-metalworking/Basic-backyard-forge
I used to have a tire rim forge. I traded a small anvil for it some years ago. It had a real coal forge duck nest (fire pot) installed in it and an electric blower with variable speed. The outside of the fire pot was packed with some sort of white clay for insulation. You could build a similar forge using the hand crank blower you have. You might want to create a firepot out of some clay or castable refractory.

I pulled the fire pot out and bult a solid fuel forge around it.
.JPG)
"Ah, but a man's reach should exceed his grasp,
Or what's a heaven for?"
Andrea del Sarto by Robert Browning
 8
8




Kevin Olson wrote:
Joshua -
Thanks for the photos of your old setup. Yes, that's more or less what I was thinking, but I'll need to make the duck nest from fire brick or something, I imagine. I was intending to use a brake drum, because I know that cast is more heat resistant than high carbon steel.
Another acquaintance built what he styled a "Viking forge", which was basically a wooden box, with a thick lining of wood ashes. He claimed that this was typical for iron age Norse forges. I don't know much about the history, so I was happy to take his word for it, at least provisionally. His master's degree was in industrial archaeology, and he was "into" experimental archaeology (attempt to replicate past historical technological achievements, I think - that's based on my observation of his activities). In any case, he made this setup work for a number of years, though he eventually switched over to a propane forge when his life got busy with a wife and children. Unlike coal or charcoal, propane heats up pretty quickly, and is cools off quickly, too. So, I know simple can work, if the operator is skilled (or maybe just determined), but given my lack of skill, I am hoping a slightly more auspicious setup will sooner yield acceptable results!
My end goal is to be able to do minor tool building and repairs, and make use of a lot of metal which gets sent out for scrap. A lot of springs, broken axles and the like just get hauled off to the big scrap recyclers. That's fine, but I figure it's better to try to use some of that stuff one or two more times before is gets turned into some commodity melt, somewhere.
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 8
8




Joshua States wrote:I would not recommend putting iron sand into a crucible. It boils out of the crucible and makes a gigantic mess in the furnace. Ask me how I know.

How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 9
9




Nancy Reading wrote:
Now I have to ask! I'm suspecting it went horribly wrong for you? - but if you tell me what you did, I may see another way around it :)
Separating the sand with a magnet is a pretty easy first step. At this point I'm more interested in achieving a metal, than what to do with it afterwards. Maybe it will be a knife blade (that would be pretty cool) but to be honest, I'd be pretty happy with a paperweight at this point. It depends on what I can achieve.
I'd really like a low tech approach and the bloomery process is pretty basic. I think the temperatures typically achieved do not go high enough to melt the iron, and I suspect that the 1100 C of a rocket stove will be enough heat to get a lumpy mass like a bloom or tamahagane.

tamahagane
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 8
8




Joshua states wrote: I got the not-so-bright idea of putting a kg of sand and some charcoal into a crucible to see what happened.
What happened is called a "carbon boil" where the sand starts to melt rapidly and all the various stuff that isn't iron starts turning to gasses and liquids. It came out of the crucible (open top) and I shut down the furnace. You may have better luck at lower temps.

How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 3
3




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 4
4




How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 8
8




Moderator, Treatment Free Beekeepers group on Facebook.
https://www.facebook.com/groups/treatmentfreebeekeepers/

















 5
5





How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 7
7




Nancy Reading wrote:I'm sort of hoping that by mixing charcoal and ore sand in the crucible I will get the mixing and the atmosphere. According to engineering toolbox again the spontaneous ignition temperature of charcoal is 349 C . Hmm, maybe that means it will all have burned away before the iron compound is hot enough to react with the Carbon Monoxide? Alternatively, the CO might be heavy enough to stay in the crucible. Maybe I'll need a lid, maybe it won't work. As I wrote above, I couldn't find references to anyone who has tried smelting in a rocket. When people use crucibles they usually get hot enough to melt the iron, so closer to 1400C than 1100C, so it might be I'll end up with nothing more than hot sand
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 8
8




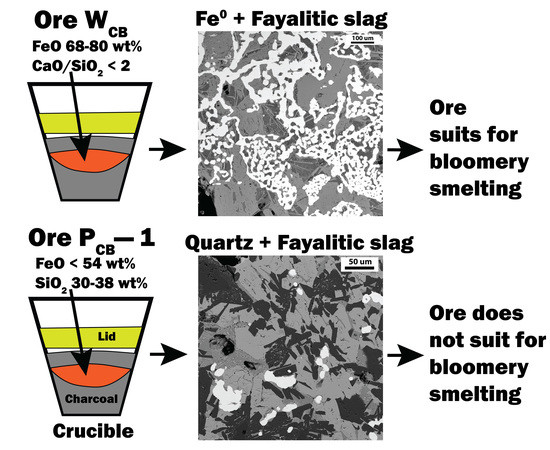
Joshua States wrote: There are a couple of videos of an open-top crucible in my furnace at this link: https://drive.google.com/drive/folders/1O7d9C1EAGKvgq4UqjYcd7wgDcOfEshis?usp=sharing
You can see the molten glass is bubbling as the gasses from the melting charge escape through the glass.

 :
:
How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 10
10




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 11
11




How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "

 9
9
















 8
8




Eino Kenttä wrote:Your plan is to use charcoal for the smelt, right? If I remember correctly, a charcoal smelt is (at least partially) self-fluxing, since the ash component in the char contains calcium. I think that's for a bloomery, though, where you use (I'd guess) more char per ore, since it's both the fuel and the source of carbon monoxide. I don't know if it'd be the same in a crucible, maybe there won't be enough ash? Don't know.
 I'm prepared for it needing a bit of experimentation.
I'm prepared for it needing a bit of experimentation.How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "












 10
10




How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 8
8




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 7
7




Joshua States wrote:Borax is the traditional blacksmith/smelting flux. You can usually find it in the laundry soap aisle of the grocery store.
How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "

 10
10




Nancy Reading wrote:
Joshua States wrote:Borax is the traditional blacksmith/smelting flux. You can usually find it in the laundry soap aisle of the grocery store.
Not in Europe though :) It is considered too toxic for general use here. I think it is a more modern development for smelting. Although does seem to have been used for joining metals, it was in short supply in industrialised countries until the 19th century (ref I'll see how I get on. I can order some online once I've had a trial run or two, if it looks like things aren't working well.
 11
11




Eino Kenttä wrote:
Nancy Reading wrote:
Joshua States wrote:Borax is the traditional blacksmith/smelting flux. You can usually find it in the laundry soap aisle of the grocery store.
Not in Europe though :) It is considered too toxic for general use here. I think it is a more modern development for smelting. Although does seem to have been used for joining metals, it was in short supply in industrialised countries until the 19th century (ref I'll see how I get on. I can order some online once I've had a trial run or two, if it looks like things aren't working well.
Even for forge welding, there are alternative/older techniques. I think my brother, who attended a blacksmithing course, said they used silica sand instead of borax, somehow. I'll have to ask him for details, but as I understood it, you'd add silica sand between the pieces you want to join (like you would add borax) but rather than evaporating it will melt, somehow facilitate the joining of the metal pieces, and then get pushed out as you hammer. I understood that it's a bit trickier than borax, but doable.
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 7
7




In a sealed crucible, a little charcoal over the top of the charge directly under the lid will burn up most of the oxygen leaving the iron to attract the carbon and any alloying elements added to the charge.
How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 7
7




In a sealed crucible, a little charcoal over the top of the charge directly under the lid will burn up most of the oxygen leaving the iron to attract the carbon and any alloying elements added to the charge.
Nancy Reading wrote: I think it was one of the ones trying to produce Damascus steel from Damascus ore...
I have no idea what "Damascus ore" could possibly be. Could you please clarify before I go full-on rant about what "Damascus" really is and how the name has evolved into something completely different from what it originally was?
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 7
7




I have no idea what "Damascus ore" could possibly be. Could you please clarify before I go full-on rant about what "Damascus" really is and how the name has evolved into something completely different from what it originally was?
How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 1
1




Nancy Reading wrote:
It was ore from a mine in Syria, supposedly with similar alloy compositions to historic sword steels. I can try and find the video reference if you like? It was US forgers that were smelting it.
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 5
5




Joshua States wrote:
Nancy Reading wrote:
It was ore from a mine in Syria, supposedly with similar alloy compositions to historic sword steels. I can try and find the video reference if you like? It was US forgers that were smelting it.
If you have the video link handy, that would be great.
Warning: rant is brewing

How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 6
6




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”
 11
11










“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 8
8




How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "
 9
9




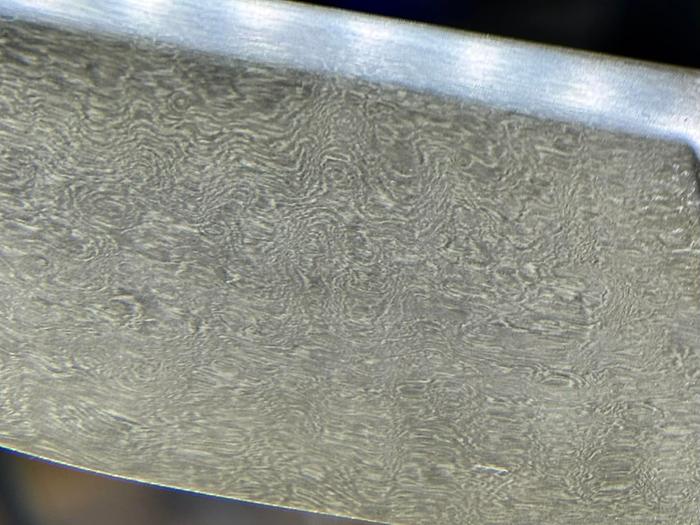
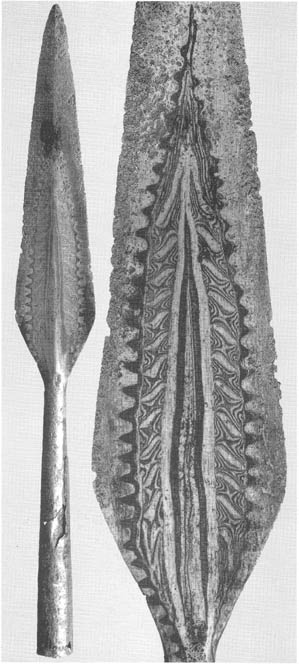
Nancy Reading wrote:I'm very happy to have the detour into historic steel terminology, thank you Joshua. It is all so beautiful, and intriguing that you can get superficially similar effects from very different processes. Those blades (all of them) are truly works of art - stunning!....I have to admit at this stage I would be fairly happy with a paperweight; if I can make a lump of metal at all that will be great!
“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”
 8
8




“So I'm lightin' out for the territory, ahead of the scared and the weak and the mean spirited, because Aunt Sally is fixin’ to adopt me and civilize me, and I can't stand it. I've been there before.”












 9
9




Joshua States wrote:That post is now live: https://permies.com/t/274766/pep-metalworking/Refining-carbon-steel
I suggest you build something similar to do your initial smelt of sand into metal.
How Permies works: https://permies.com/wiki/34193/permies-works-links-threads
My projects on Skye: The tree field, Growing and landracing, perennial polycultures, "Don't dream it - be it! "

|
What we've got here is a failure to communicate. The solution is in this tiny ad:
permaculture thorns, A Book About Trying to Build Permaculture Community - draft eBook
https://permies.com/wiki/123760/permaculture-thorns-Book-Build-Permaculture
|