I did not find the right forum to place this thread? At least it has to do with water.
For Paul:
Again referring to the Q&A Podcast with Geoff Lawton:
You wanted to know more about water harvesting by condensation and how effective stone piles are for this.
I will try to answer it.
We have to do some theory first.
First we need to know:
How much water is in your air?
To check this, you have to measure the air temperature and the relative air humidity (by a hygrometer).
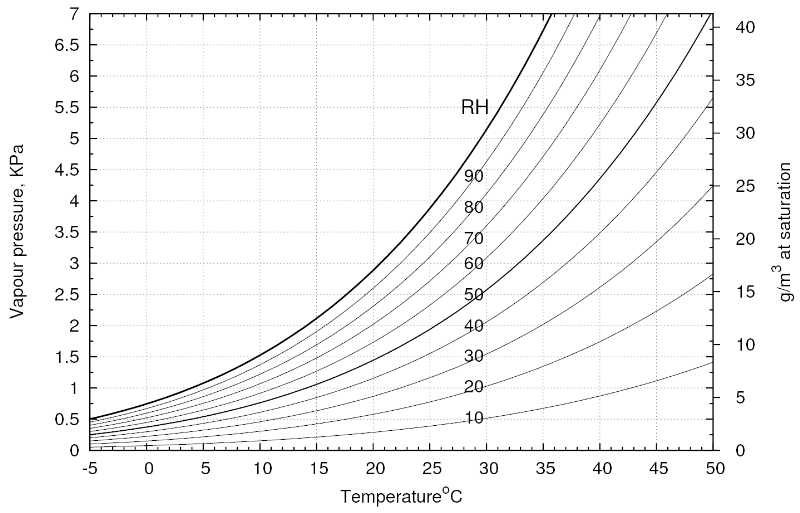
Than you can use this relative air humidity diagram (at standard pressure) to find out how much water is available.
For example:
If you have 20°C and 50% RH (relative humidity) you go to 20°C on the lower axis. From there you go up to the 50% RH line and from the point you cross this line you go horizontally to the right axis, showing you the water content. In our case we have approximately 8.5 g water per 1 m3 of air.
If you have 20°C and 100% RH there is about 17 g water per 1 m3 of air.
The higher the air temperature and the higher the RH, the more water is in the air.
The 100% RH curve shows us, how much water vapor the air can maximally take up at a certain temperature.
As you see in the diagram, cold air does contain very little water, even at 100% RH.
Therefore water harvesting by condensation is betty unattractive in an environment with cold, dry air. Of course you can freeze out some moisture there, but it is technically complicated and has a low yield.
When you have warm air with high RH, there is a lot of water that can be harvested, if you can create a surface cold enough to cause condensation.
The next thing to know is: How do we get this water out of the air and how much of it?
Let us start with the 20°C and 50% RH again.
Go to 20° C and up to the 50% RH line.
Now we have to cool this air down to the point where we reach 100% RH.
Therefore in the diagram you draw a horizontal line to the left, until you reach the 100% RH curve.
From there you draw a vertical line down to the temperature axis. We debark at about 9°C.
This tells us: If we have air of 20°C and 50% RH and bring it in contact with a surface of 9°C, it will cool down to its 100% humidity holding capacity and condensation will begin. We have reached the dew point.
The problem is: As soon as one drop of condensation occurred, our 9°C air contains less water and is again below the 100% RH curve. The condensation stops.
If we want some yield, we have to make our air even cooler, to create more condensation.
What if we could cool it to 5°C?
We start with our example again:
20°C
Up to the 50% RH line.
Horizontal line to the right Axis: We have 8.5 g of water in every 1m3 of air.
Horizontal line to the left, until we reach the 100% RH line at about 9°C. Condensation starts.
Then we follow the 100%RH line down to 5°C. More and more condensation occurs.
From there we draw a horizontal line to the right axis again.
There we see, that we have about 6.5 g of water left in each m3 of air.
Now we can calculate our yield:
8.5 g at the beginning. 6.5 g left at the end. Our possible yield is about 2 g from each m3 of 20°C 50% RH air.
(In reality it is a bit more, as the air contracts too, when cooling down.
If we want to go into this detail, we can use the Gay-Lussac low:
V = V0 x T / T0 = 1m3 x (5+273.15)K / (20+273.15)K = 0.95 m3
(The volume of 1 m3 air shrinks to 0.95 m3, while we are cooling it from 20°C to 5°C.)
So we get a possible yield of 8.5 g – 0.95 *6.5 g = 2.32 g)
One more example:
If we start with air of 20°C and 100% RH, we get condensation immediately after we start cooling.
20°C
Up to the 100% RH line.
Horizontal line to the right axis ->17 g of water.
Then we follow the 100% RH line down to 5°C.
Horizontal line to the right axis ->6.5 g of water.
Possible water yield per 1m3 of warm air: 17 g – 0.95*6,5g = 10,8 g
I hope you get the picture: The warmer the air, the higher the RH and the colder your condensation surface, the more water you can harvest.
On the other hand, if you have cold, dry air, let us say 10°C and 20% RH, you need a surface temperature far below 0°C to get any condensation (any hoarfrost) at all.
This heads us to the practical issues of condensation water harvesting.
What air conditions do you have and how cold can you make your stone pile?
Up in an arid mountain region with dry air you might be happy to get some hoarfrost on the outside of the pile while the inside stays warmer than the air flowing through and therefore will not condensate any water at all.
Close to a warm ocean ore lake, which is producing hot air with high humidity (let us say 30°C, 90% RH) a huge pile of stones that has a low temperature of 15°C inside, can condensate a lot of water if you somehow manage to make the warm air flow through the pile.
But: The heat of condensation has to go somewhere.
Each liter of water condensing delivers about 2.3 MJ = 2300 kJ (depending on the condensation Temperature) of energy to your condensation system.
Your condenser is heating up while working. And the warmer it gets, the lower its effectiveness.
If you use a big stone pile, you can stop the airflow and let it cool down to ground temperature (like a root cellar) again. Or you have to reduce the flow of warm air to a level, that allows the pile do keep low, stable temperature in exchange with the soil below it.
Trees are more effective condensers. They use a different technique. In the night, their leaves cool down very fast as they are radiating their warmth into the nightly sky.
This rapid cooling allows them to start condensation soon (assumed the air is warm and moist enough). As they keep radiating warmth all night, the warmth they trap from condensation does not increase their temperature much.
And leaves have one more advantage: They can take up at least part of the condensed water thorough their stomata.
That is the next problem with condensation water harvesting:
You have a nice cold surface at night which is condensing water. But if the water drops stay on this surface they will be evaporated by sun or wind in the morning within a short time and you have won nothing.
You not only have to condense the water, you have to collect it and store it somewhere, protected against sun and wind.
There have already been invented lots of technical condensation water harvesting systems.
Most of them are imitating leafs. They use some thin fabric or thin sheet metal that is cooling down very fast in the evening. An angular array is more effective for cooling down than a vertical one.
But the more vertical it is, the easier it is to make the condensed water to run off into storage.
One could perhaps use some lotus effect cover to accelerate the run off.
(I saw you are using profiled sheeting on some of your buildings. Check these out. Usually they are good nightly condensers if your air is warm and moist enough).
Search for radiative condenser.
The
air well constructions made of heavy stone piles you mentioned (like Zibold´s dew condenser) to not work under most conditions (as they do not get cold enough in the inside). Or they have very little yield, hardly justifying the effort to build them. Only in the right place they will make some sense.
And there are some relatively new interesting developments like the airdrop irrigation system:
http://www.gizmag.com/airdrop-wins-james-dyson-award/20471/
As Geoff said: You could write a whole book on this topic alone.

 1
1