
 1
1








Sometimes the answer is nothing





wayne fajkus wrote:Have you checked ph of rainwater separate from what is in the storage tank?
It does not sound alarming to me. I would not drain it considering it is only being used for laundry. I don't have the benefit of the rain you have though. It could be weeks for another rain. That plays a factor.





Some places need to be wild
 3
3




Eric Hanson wrote:Andy,
I suppose that if alkalinity were a problem you could add just a little vinegar and check the ph again. Is alkaline water going to be a problem? Rainwater is naturally slightly acidic, so if the problem is caused by baking soda, the problem may resolve itself over time.
Please, let me know your thoughts,
Eric
 1
1




Standing on the shoulders of giants. Giants with dirt under their nails
 1
1




Standing on the shoulders of giants. Giants with dirt under their nails
 1
1





'Every time I learn something new, it pushes some old stuff out of my brain.'
 5
5




To lead a tranquil life, mind your own business and work with your hands.
 2
2




Lina
https://catsandcardamom.com
 6
6




Iterations are fine, we don't have to be perfect
My 2nd Location:Florida HardinessZone:10 AHS:10 GDD:8500 Rainfall:2in/mth winter, 8in/mth summer, Soil:Sand pH8 Flat
 1
1




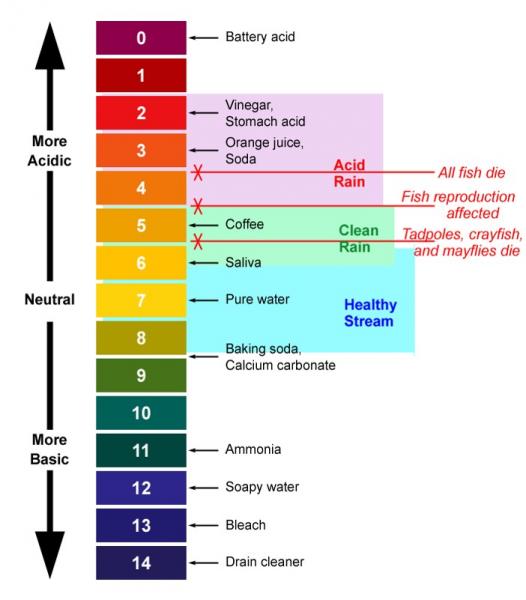
Mandy Launchbury-Rainey wrote:Please be carefull and remember that pH of 10 is 1000 times more alkaline than neutral.
Your friend isn't always right and your enemy isn't always wrong.






Iterations are fine, we don't have to be perfect
My 2nd Location:Florida HardinessZone:10 AHS:10 GDD:8500 Rainfall:2in/mth winter, 8in/mth summer, Soil:Sand pH8 Flat




This is all just my opinion based on a flawed memory

 1
1




Ben Zumeta wrote:run it through a bunch of woodchips or biochar and see if it comes through safe for irrigation.
Your friend isn't always right and your enemy isn't always wrong.




This is all just my opinion based on a flawed memory







Iterations are fine, we don't have to be perfect
My 2nd Location:Florida HardinessZone:10 AHS:10 GDD:8500 Rainfall:2in/mth winter, 8in/mth summer, Soil:Sand pH8 Flat




 1
1




 1
1




This is all just my opinion based on a flawed memory







Iterations are fine, we don't have to be perfect
My 2nd Location:Florida HardinessZone:10 AHS:10 GDD:8500 Rainfall:2in/mth winter, 8in/mth summer, Soil:Sand pH8 Flat

| I agree. Here's the link: http://stoves2.com |

